Polyphenylene sulfide (PPS)
PPS is an organic polymer consisting of aromatic rings linked with sulphides. Synthetic fiber and textiles derived from this polymer are known to resist chemical and thermal attack at up to 240°C. Rigid and opaque, PPS is used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes and gaskets.
- History
- Properties
- Applications
- Processes
- Recycling
- Faq
History
1888: PPS was uncovered by Friedel and Crafts
1973: commercial process for polymerizing was successfully developed and manufactured by Phillips Petroleum (USA)
Early versions of PPS had a relatively low molecular weight so applications were developed to use it in specialty coatings. However, once the molecular weight was increased by a thermal cross-linking reaction in the presence of oxygen, processing and mechanical properties were improved. Once it was discovered that PPS would be suitable for injection molding and had excellent heat and chemical resistance, demand increased, especially for more advanced engineering component applications.
Properties
PPS can be molded, extruded or machined to high tolerances.
In its pure solid form, it may be opaque white to light tan in color.
Maximum service temperature is 218 °C.
PPS has not been found to dissolve in any solvent at temperatures below about 200°C.
An easy way to identify the plastic is by the metallic sound it makes when struck.
Applications
PPS products have been specified in a wide range of injection mold applications including:
- under-the-hood automotive parts
- power train components
- pumps
- fuel system components
- surface mounts

They are also used in:
- electrical/electronic components
- blower and pump parts
- protective and non-stick coatings
- surgical devices
- power tools
- PPS which is formed by blow molding or extrusion can be used to produce films and composites, and is an excellent candidate for fuel cells and diesel fuel cars.
Processes
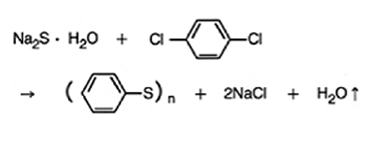
PPS can be polymerized by the polycondensation reaction of para-dichlorobenzene (p-DCB) and sodium sulfide (Na2S) or sodium hydro sulfide (NaSH) in a polar solvent under elevated temperature and pressure conditions.PPS is created by combining various elements, including a dehydration reaction, sodium chloride removal reaction, exothermic reaction, and high temperature pressurization reaction.
The polymer in the diagram below is processed as a molding compound after going through a refining process and thermal cross-linking treatment. This results in branched PPS.
Recycling
PPS applications are 100% recyclable, either mechanically or as feedstock, and energy from waste can be recovered at incineration plants.
The most appropriate recovery options depend on numerous conditions. These include local legislation, plastics part design, access to sorting facilities and regional logistics and recycling costs.
