Polyvinylidene fluoride (PVDF)
PVDF is a family of semi-crystalline fluoropolymers. They possess the characteristic stability of fluoropolymers especially when exposed to harsh thermal, chemical and UV environments.
This polymer has two important properties. Firstly, the polymer's polymorphism, and secondly its piezoelectric properties (when crystals generate electrical energy when mechanical stress is applied). It is the later which makes this polymer ideal for tactile sensor arrays, low cost strain gauges and lightweight audio transducers.
- History
- Properties
- Applications
- Processes
- Recycling
- Faq
History
1969: Dr. Heiji Kawai discovered the piezoelectric properties of PVDF.
1981: Furakawa and Johnson confirmed PVDF’s piezoelectric nature and identified the Curie point of 103ºC. (The Curie point is the temperature above which the piezoelectric effect breaks down.)
Properties
PVDF has a very high purity and is chemical intert to most acids, aliphatic and aromatic organic compounds, chlorinated solvents and alcohols.
It is highly abrasion resistant, compared to that of polyamides and has a low coefficient of friction.
PVDF can be used within a wide range of temperatures (making it an excellent fire resistant), is unaffected by UV light and has a strong resistance to radiation. PVDF has a good capacity for thermoforming and can very easily be joined by welding.
Applications
PVDF is used in a multitude of applications across:
- aerospace
- biotechnology
- electronics industries (e.g. robotics, sensors and electrical wire insulation)

It is also used to make:
- hollow fibres
- flat sheet
- tubular membranes for the medical and food beverage industry
Processes
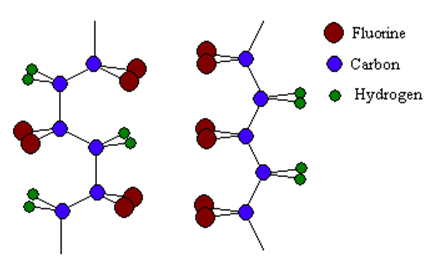
PVDF (polyvinylidene fluoride) is produced by the polymerization of vinylidene difluoride. PVDF exists in four forms or crystal phases - alpha, beta, gamma and delta.
See the following diagram for the structure of AlphaPVDF (left) and BetaPVDF (right).
Recycling
PVDF is recyclable and can be used for energy recovery.
