Polybutylene Terephthalate (PBT)
PBT is a thermoplastic crystalline polymer which is mechanically strong, solvent resistant and has low shrinkage properties during formation. PBT is also heat resistant up to 150°C (or 200°C if it is reinforced with glass fibre). It can be treated with flame retardants to make it noncombustible.
- History
- Properties
- Applications
- Processes
- Recycling
- Faq
History
1932: first fibre-forming polyesters from Carothers und Hill (DuPont) made out of aliphatic dicarboxylic acid and aliphatic diols.
1942: first PBT fibres from Schlack.
1970s: market launch of PBT compounds.
Properties
PBT is used for its:
- high rigidity and strength
- very good dimensional stability
- low water absorption
- high resistance to many chemicals
Moreover, PBT exhibits:
- exceptional resistance to weathering
- excellent heat aging behaviour
Applications
Automotive: headlights, wipers.

Industry applications: Electronic Stability Programme (ESP) control modules, gear housing, steering-angle sendors, door control devices or airbag connectors.

Universal applications: Strips for window frame profiles, fibre optical cables.
Processes
PBT is produced by polycondensation of terephthalic acid or dimethyl terephthalate with 1,4 –butanediol using special catalysts.
Terephthalic acid, dimethyl terephthalate and 1,4 -butanediol are obtained from petrochemical feedstocks, such as xylene and acetylene. The chemical structure is illustrated in the following structural formula:
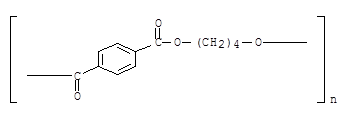
Recycling
PBT is 100% mechanically and feedstock recyclable. It can also be recovered in state-of-the-art incineration plants by generating energy. The most appropriate recovery options depend on many boundary conditions such as local legislation, plastics part design, access to sorting facilities and regional logistics and recycling costs.
